New Ligands and Complexes for Homogeneous Catalysis
New Ligands and Complexes for Homogeneous Catalysis
The design of novel ligand and metal catalysts has enormous ramification for chemical syntheses and industrial processes. Our work has impact in the understanding of metal-ligand reactivity and the control of industrially-relevant processes such as olefin and lactide polymerisation.
New Ligands and Complexes for Homogeneous Catalysis
Isotacticity and Rate Control in Lactide Polymerisation
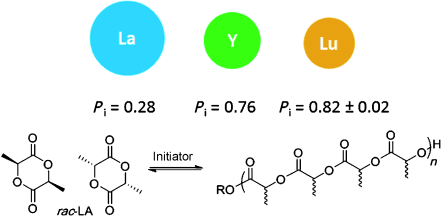
Via collaboration with Prof. Charlotte Williams, we have developed a new class of ancillary ligand: an iminophosphorane derivative of the popular “salen” ligand, termed “phosphasalen”. This versatile ligand framework allows for much functionalization and derivation, and lends itself to the development of families of metal pro-catalysts from which meaningful trends and data can be elucidated. Within lactide polymerisation, one of the greatest challenges is to engineer some polymer tacticity control via the metal catalyst. We have demonstrated that yttrium phosphasalen initiators are highly active for the stereocontrolled ring-opening polymerization, and they enable access to high iso-selectivities (Pi = 0.84) or hetero-selectivities (Ps = 0.87). The initiators also show very high rates, excellent polymerization control, and tolerance to low loadings; furthermore, no chiral auxiliaries/ligands are needed for the stereocontrol. The combination of such high rates with high iso-selectivities is very unusual.
Key references: Organometallics, 2012, 31, 4729; J. Am. Chem. Soc., 2012, 134, 20577; Inorg. Chem., 2013, 52, 12561; Organometallics, 2013, 32, 1475; Angew. Chem. Int. Ed., 2014, 53, 9226; Dalton Trans., 2015, 44, 12326
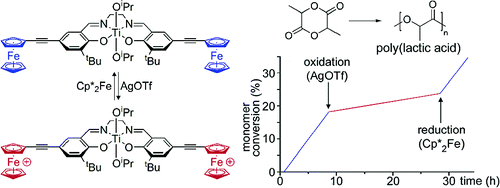
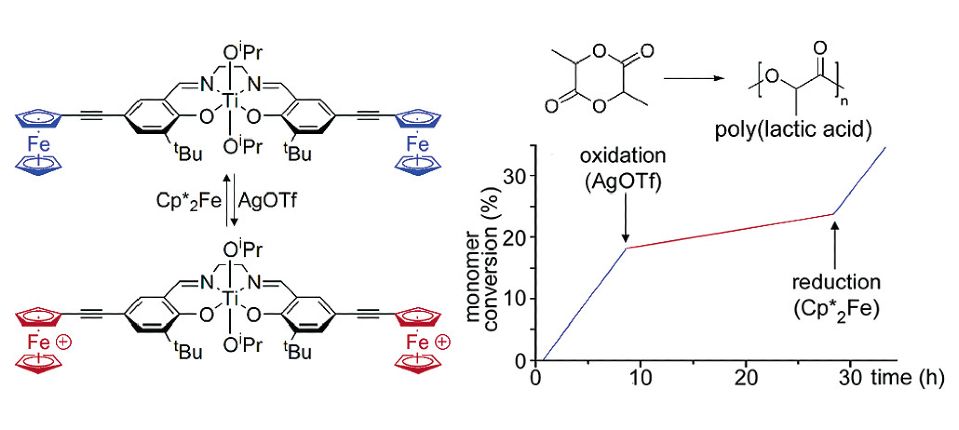
Redox-Active Catalysis
In a proof of concept study, we have established for the first time that redox switches may be used to attenuate the activity of single-site polymerization catalysts. Thus, a titanium-based lactide polymerization initiator supported by a ferrocenyl-substituted salen ligand exhibits a substantially higher rate of propagation than its oxidized dicationic ferrocenium analogue. The reversibility of the redox event is demonstrated by treatment with chemical redox reagents.
Key references: J. Am. Chem. Soc., 2006, 128, 7410; New J. Chem., 2011, 35, 2162; Chem. Commun., 2013, 49, 5663; Dalton Trans., 2013, 42, 2813; Dalton Trans., 2019, 48, 72