DREAM: Drug-Resistant Epilepsy Ambulatory Monitoring
Current Project (2023-2025)
Research Team: Timothy Constandinou (PI), Ian Williams, Peilong Feng, Adrien Rapeaux.
Project Partners: Dorian Haci (Co-I, MintNeuro), Jonathan Casey (MintNeuro), Antonio Valentin (Co-PI, King's College London), and Ioannis Stavropoulos (King's College London).
Funding: National Institute for Health and Care Research (NIHR) Invention for Innovation (i4i) Product Development Award (PDA)
Background
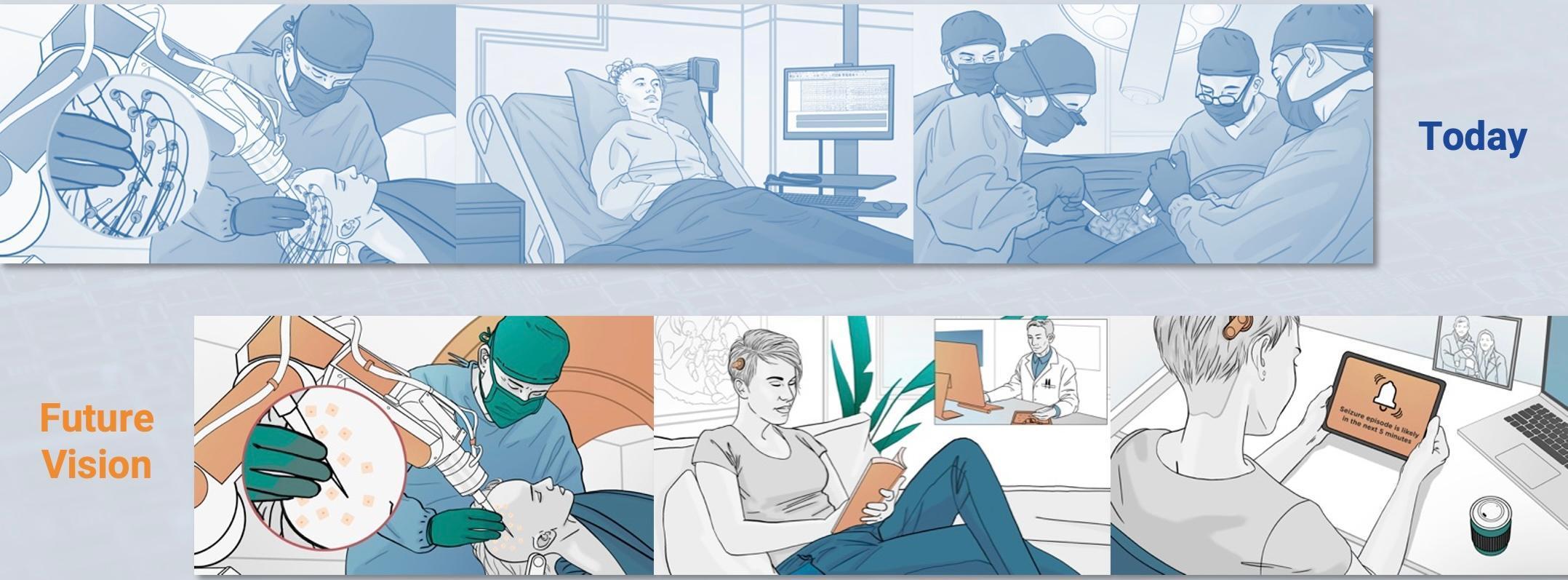
Around 600,000 people in the UK have epilepsy. Roughly a third of them cannot control their seizures with medication and are diagnosed with Drug Resistant Epilepsy (DRE). In the subsequent 2 years, it is likely that around 10% these patients will die; and around 1% of those living with DRE will die each year in sudden unexpected deaths, often during sleep. DRE is associated with increased life challenges resulting in lower social outcomes (e.g. educational achievement, employment status, reduced likelihood of long-term relationships), and patients suffer disproportionately from traumatic injuries (e.g. fractures, burns) and psychiatric diseases. Surgery could help many, typically by removing part of the brain that is involved in initiating the seizures. However, it is often necessary to perform invasive brain activity recordings to identify which part(s) of their brain are initiating seizures. This is achieved by boring tiny holes in the patient's skull and inserting electrodes deep into the brain, the electrodes are connected to wires running through the skull and skin and connect to equipment that records the brain signals. If the patient has a seizure, the recordings can show which parts of the brain were first affected and associated with the start of a seizure.
The recording process has 2 key downsides:
- The patient has an open wound for the monitoring period. The associated risks limit the monitoring period to around 3-weeks and limit the amount of data gathered (or not observing any seizures) which can prevent surgery.
- The patients are tethered to a hospital bed: this can be deeply distressing for patients, who are constrained even from trips to the bathroom, and is sometimes not tolerable for small children.
Our Approach
There is currently no escaping the need for electrodes and wires to be inserted into the brain, however, our vision in the future is to connect each of these electrodes to a small microchip implanted under the skin. This small change enables a fundamental difference for the patient: the clinician can close up the skin after implantation, greatly reducing the risk of infection and greatly increasing the potential recording time. We will also create a small wearable device that wirelessly receives data from the implanted microchip(s), untethering the patient, enabling them to leave not just the hospital bed, but the hospital itself, and to return to their daily life for the monitoring period.
Delivering this vision requires 2 phases: the first phase (covered by this i4i PDA) demonstrates the feasibility of the device by performing a First-in- Human trial where the microchip is not implanted but instead sits alongside hospital equipment; the second future phase would then repackage the phase-1 system creating the wearable readout device and encapsulating the microchip for implantation.