Research
The Solar Coatings Group work on a range of solar-state materials that can harvest sunlight to drive a wide range of chemical processes for solar fuels production and remediating the environment
The chemical processes studied include:
- the conversion of H2O into O2 and clean H2 fuel
- the conversion of CO2 waste streams into carbon-based products/ feedstocks (including syngas, HCOOH, CH4, etc.)
- the conversion of NOx pollution in city air into benign products (including NO3- or N2)
- the removal of heavy metal arsenic from water
- the disinfection of surfaces and contaminated water streams, removing bacteria and viruses
The Solar Coatings Group use various strategies to study, develop and prototype solid-state materials
Our strategies include:
- the use of in operando spectroscopies for the time-resolved study of reaction kinetics and mechanisms, which are used to inform the development of rational materials design strategies
- the development of scalable materials synthesis strategies and their optimisation to enhance material functionality, which includes the use of combinatorial materials synthesis, high-throughput physical and functional characterisation and machine learning methods to more rapidly identify promising materials and their optimisation
- the scale up of promising materials syntheses for the production of prototypes at scales commensurate to their desired commercial application, and the implementation of technoeconomic and life-cycle analyses to inform improved prototype design
The Solar Coatings Group collaborates with various research groups across Imperial College London, the UK and internationally to conduct cutting edge research in the use of sunlight to drive chemical conversions
Our collaborators include:
- The Durrant Group and Bakulin Group - for time-resolved spectroscopy studies of reaction kinetics and mechanisms
- The Stephens Group - for studies on the electrochemical conversion of H2O
- The Hankin Group - for the testing of large-scale (~100 cm2) photoelectrochemical prototypes
- The Nelson Group - modelling photoelectrochemical device performance
- The Walsh Group and Ganose Group - for the use of machine learning tools to identify relationships from the big data generated in our combinatorial research
- The Harrison Group - for the use of computation to study the electronic interactions found at the interfaces in our solid-state structures
- The Myers Group - for the fabrication of prototype building materials that incorporate our solid-state catalysts and use of technoeconomic and life-cycle analyses to inform improved design
- The Weiss Group - for the development and testing of photocatalytic materials for arsenic removal from polluted waters
- The Carmalt, Parkin and Blackman Groups at University College London - for the development of photoelectrodes for water splitting
- The Sotelo Vázquez Group at the Universidad Rey Juan Carlos - for disinfection testing of surfaces and contaminated water streams
- The Sathasivam Group at London South Bank University - for combinatorial studies of transparent electrode materials
- The Tittl Group at Ludwig-Maximilians-Universität München - for the development of novel plasmonic photocatalysts for energy and the environment
Ongoing research projects in the Solar Coatings Group
CO2 Reduction - Can We Convert CO2 Into Useful Chemicals Using Sunlight? Developing Copper Iron Oxide Based Photoelectrodes For CO2 Conversion
The release of CO2 from mankind’s excessive use of fossil fuels is the primary cause of Global Warming, which has resulted in pervasive and lasting damage to the earth’s climate and ecosystems. Photosynthesis is nature’s example of how sunlight can renewably produce energy rich chemicals from CO2, and has inspired artificial approaches for how we can convert our CO2 waste into useful chemicals. Many materials have been explored for this purpose, with a copper iron oxide (CuFeO2/ CuO) based system showing the highest solar-to-chemical conversion of any photoelectrochemical system to date.


For this photoelectrode to be applied in commercial devices, it should be produced using a scalable method. During this project, we will explore the fabrication of copper iron oxide and copper oxide based photoelectrodes using a scalable process, chemical vapour deposition (CVD). We will physically characterise the two system components and the full device using a suite of methods (XRD, UV-visible absorption spectroscopy, SEM, AFM, XPS etc.). We will then measure their capability of converting CO2 into chemicals using a photoelectrochemical set-up to optimize and match the photoelectrodes’ performance, and study the products via a connected gas chromatograph (GC). Thus far, this system has primarily driven the conversion of CO2 into formate (HCOO-). During this project, we will also explore how to control the selectivity of this conversion process and drive other useful conversions (e.g. CO2 to CO, CH3OH, etc.). This may be achieved by adding co-catalysts to the surface of our materialor the electrolyte composition.
Electrochemical Water Splitting - Exploring Transition Metal Nitrides as Support Materials For Iridium-Based Electrocatalysts
Proton exchange membrane (PEM) electrolysis is the most promising technology for widespread hydrogen generation using renewable energy. However, the use of highly scarce iridium at the anode, where the kinetically sluggish oxygen evolution reaction (OER) occurs, will likely limit the mass production of PEM electrolysers to the TW level. The global production of iridium is less than 10 tonnes per year (a by-product from producing other Platinum group metals). Relatively high mass loading of iridium are required at the anode to maintain the performance over a long-period of operation in PEM electrolysers. It has been argued that around 40 years of iridium production would be needed to achieve 1 TW(H2) when operated at 2 A.cm-2 with the mass loading of 1 mg.Ir.cm-2 at an efficiency of ~64%.
Many classes of materials have been explored as alternatives to iridium, but none of them have shown the performance criteria required for them to replace iridium in acidic environment of PEM electrolysers. Another way to reduce the usage of iridium is to use a catalyst support that increases the dispersion and decreases the agglomeration of the catalyst. The most well-studied OER support materials are oxide systems, such as Sb-doped SnO2 (ATO) and TiO2, but have drawbacks. In ATO, under operating conditions, the Sb dopant is susceptible to leaching, accelerating the dissolution of Sn and corrosion of the support. And in TiO2, under operating conditions, the support was stable but OER activities were penalized by the low-conductivity of the support. Therefore, it is important to develop new corrosion resistant and conductive supports for iridium anodes in PEM electrolysers.

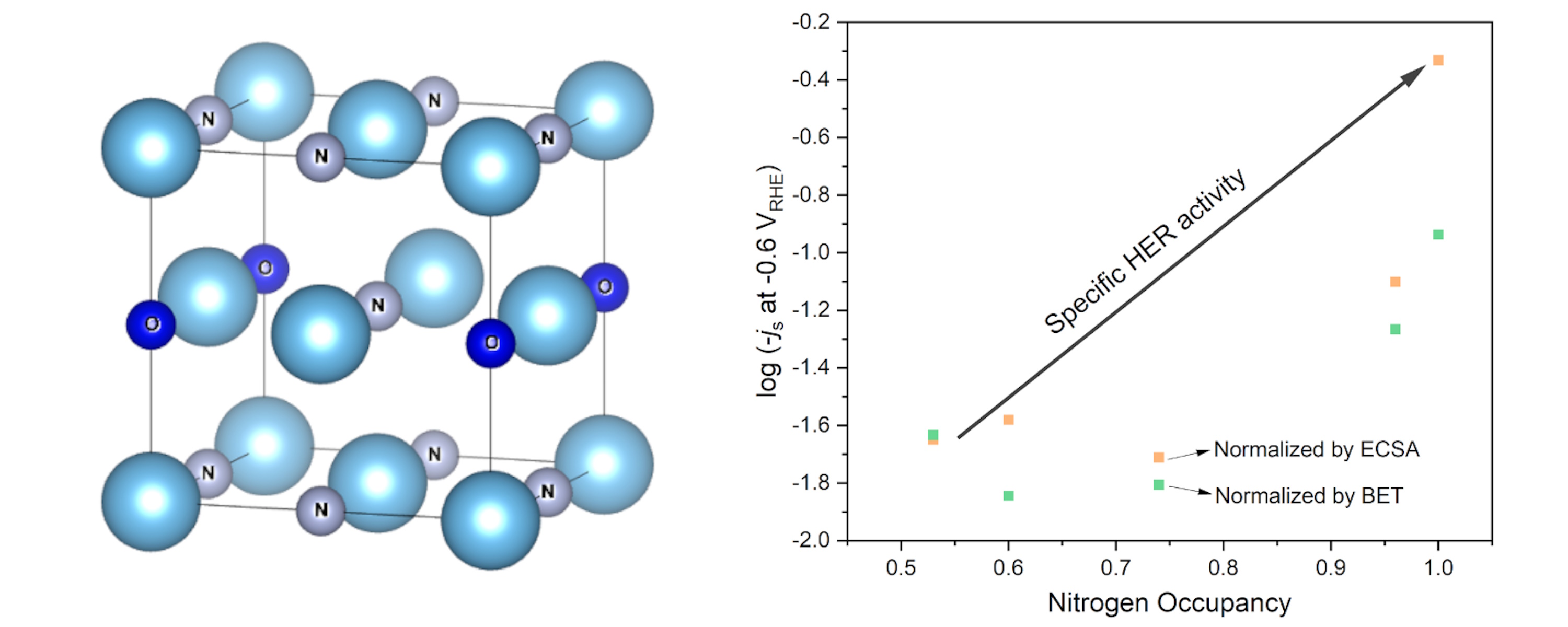
Transition metal nitrides (TMNs) have the potential to be used in electrochemical energy conversion and storage systems as they display high electrical conductivity, mechanical robustness and chemical stability. The formation of nitrides often results in an increased robustness and resistance to corrosion compared to the bare metal and metal alloys. Importantly, early transition metal nitrides can display similar electronic structures to iridium and other platinum group metals, making them an ideal support.8 Thus, a metal-support effect, which sustains lower iridium oxidation states is likely to occur between metallic transition metal nitrides and metallic iridium when they are combined. In this project, nitrides of abundant and low-cost transition metals will be explored to support iridium to catalyze OER. We will study their performance using electrochemical methods and study their structures using a range of techniques, including X-ray absorption spectroscopy (XAS).
The results from this work will inform the future design of iridium-based anodes for PEM electrolysers and likely result in a significant reduction in the cost of fabricating such a device, and therefore, commensurate to future demand.
Electrochemical Water Splitting - Exploring Transition Metal Nitrides for Low-Cost and Stable Electrocatalysts
The fastest growing renewable energy technology are photovoltaics, which can convert solar energy into electricity. However, sunlight is intermittent, and the intensity changes with the season. As the electrical energy generated by photovoltaics needs to be used at the point of generation, there is a need to develop new ways of storing the energy they produce, so that it is not wasted. Currently, there is no integrated means of storing this excess energy; however, one particularly promising approach is to convert and store the electrical energy produced by photovoltaics within chemical bonds (e.g. fuels). This has resulted in the development of electrocatalysts, which can drive useful electrochemical reactions from the electrical energy provided by photovoltaics to produce fuels.
Various materials have been explored for this purpose, where typically, platinum group metals (PGMs) have shown the greatest promise; however, these metals (e.g. Pt, Ru, Ir, Pd etc) are expensive and rare. In order to enhance the commercial viability of this strategy, alternative low-cost materials are being developed. Interestingly, early transition metal nitrides (TMNs) show a strong resemblance in their electronic properties to PGMs, and have demonstrated promising catalytic activity towards water splitting. Recent work has shown that these materials can either be used as potential replacements for PGMs, or may be able to act as a support for existing PGMs due to their high chemical resistance, meaning that less PGM may be required for such electrocatalytic technologies to function.
During this project we will develop hydrothermal and ammonolysis routes to early TMN nanopowders that have shown promise as either an electrocatalyst or electrocatalyst support for driving the water splitting reaction. We will characterise the coatings using a range of methods (X-ray diffraction, Raman spectroscopy, scanning electron microscopy etc), and examine their electrocatalytic properties towards the water oxidation reaction using electrochemical methods.
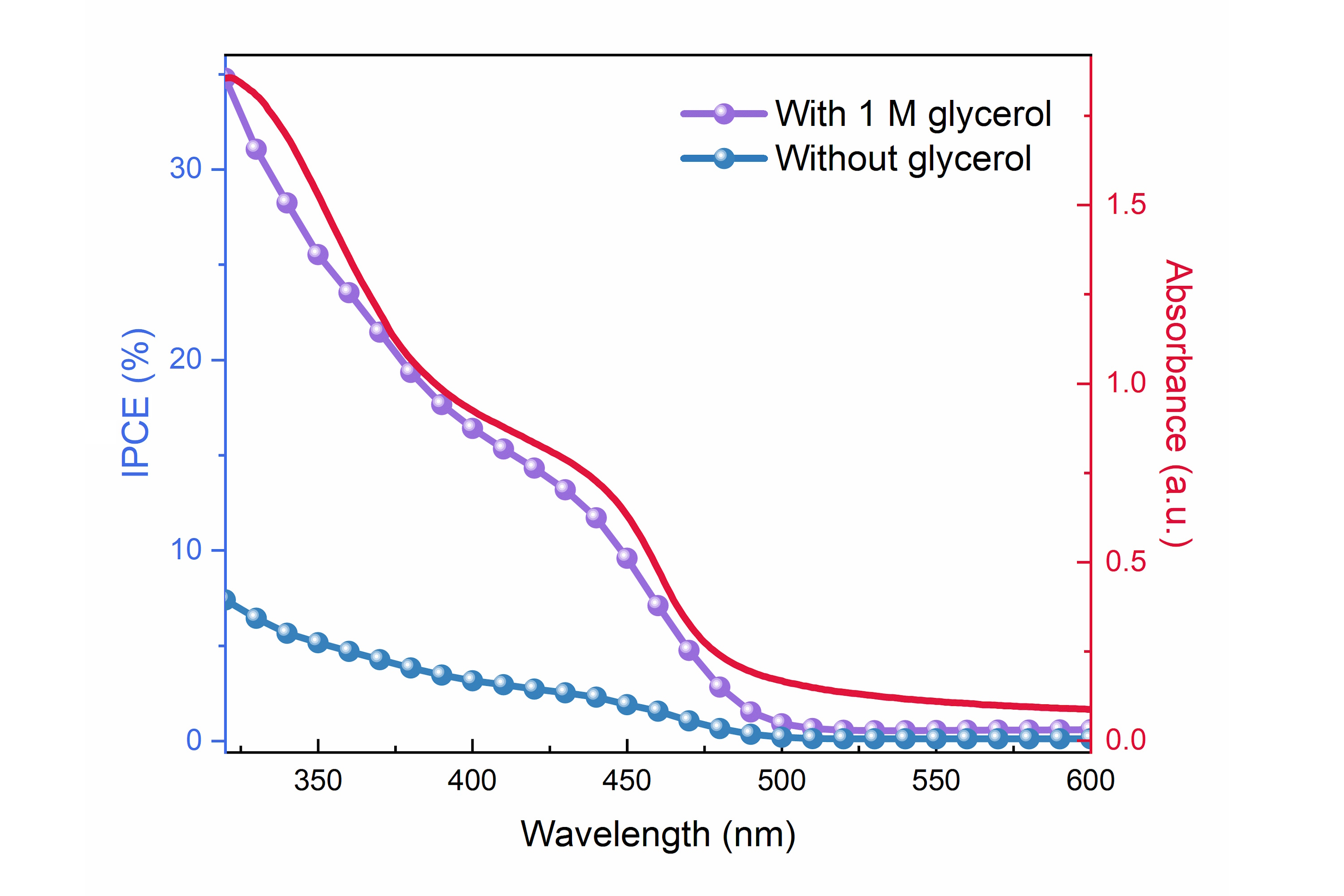
Glycerol oxidation - Tracking the Charge Carrier Dynamics in Operando Metal Oxide Heterojunctions for Added Value Oxidations
The release of CO2 from the combustion of fossil fuels is the primary cause of Global Warming, which is causing pervasive and lasting damage to the Earth’s climate and ecosystems. To mitigate the potentially catastrophic effects of climate change, an immediate and extensive reduction in CO2 emission must occur. Plant photosynthesis is nature’s example how sunlight can be used to produce renewable carbon-neutral fuels. Bio-inspired approaches (artificial photosynthesis) have shown great promise. One particularly promising approach is the solar driven photolysis of water – water splitting – to produce hydrogen fuel (a versatile fuel that burns cleanly to water with no CO2 release).
Many metal oxide (TiO2, WO3, BiVO4) semiconductors have emerged as potential candidate of water splitting. They are often quite durable, possess low toxicity and can be grown by low-cost methodologies. Recent research has shown that a very promising method, seemingly vital for producing the most efficient devices, is to couple metal oxides and form a heterojunction.
At the same time, realization of full-potential and functioning of these hetrojunction-based real photoeletrochemical cells requires knowledge on controlling key processes (like interfacial charge separation to their transport) under working conditions, which remains relatively unexplored. This would further unravel key routes to engineering this class of heterojunction architectures to boost the efficiency of the water splitting process.

During this project, we will synthesise a range of heterojunction systems and will employ advanced spectroscopic tools to find out how the type of heterojunction and variations in synthesis strategy effect the water splitting efficiency.
Interestingly, it has been found that the kinetic bottleneck of the overall water splitting reaction, water oxidation, can be circumvented by oxidising more readily oxidised compounds. This process becomes attractive if the oxidation of these compounds is selective and yields a higher value oxidation product. One potential candidate is glycerol, which has a significantly higher value when oxidised to the aldehyde or ketone. In this project, we will also explore how metal oxide photoelectrodes can be used to selectively oxidise glycerol as a value-added oxidant in the overall water splitting process. To unravel the underlying reaction mechanisms, we will study this process using in operando time-resolved spectroscopies, including pump-push photocurrent (PPP) spectroscopy that can be used to monitor the de-trappping of static charge carriers.
NOx Remediation - Developing Co-catalysts to Improve the Activity of Visible Light-Active Bismuth Oxybromide Photocatalysts for NOx Remediation
Cities are becoming increasingly challenging places to live. Emissions from car exhausts release carbon particles and NOx gases that are cause respiratory disease and cancer. In some cities, this problem is so severe that breathing city air is likened to smoking 10 cigarettes a day. In many areas of London, NO2 gas levels breach the safety limit of 200 µg.m-3 on a daily basis. NOx gases also cause problems in rural areas, as they react to form highly toxic and carcinogenic compounds that kill plants and reduce crop yields.
Materials that use sunlight to drive useful chemical processes (i.e. photocatalysts) have the potential to solve poor air quality. Sunlight is our largest source of energy; the amount reaching the Earth every hour being nearly twice the size of the world’s annual energy demand. This vast source of energy is now being harnessed by commercial products to keep surfaces clean, and also purify our air, with products such as Italcementi TX ActiveTM and TOTO HydrotectTM tiles.

Currently, all of these products rely on the light-activated material, TiO2. Although TiO2 is a highly robust, cheap and active photocatalyst, its wide bandgap limits activity; where only ultraviolet light has sufficient energy to excite the material, which accounts for <5 % of the solar spectrum in power terms. As such, new materials need to be developed that can harness more sunlight and use this light more efficiently.
Recent studies have shown that the more narrow bandgap semiconductor, BiOBr, can also oxidise NOx. In this project the student will synthesise BiOBr nanopowders, incorporate them onto a range of building materials (such as glass, tiles, cements and filters), and study the ability of these materials to remedy NOx pollution using light. Key to this project is the development of surface co-catalysts, that will be in contact with the BiOBr photocatalyst. We will explore bismuth, copper and other metallic co-catalysts to enhance the function of the BiOBr photocatalyst in remediating NOx, through the potential increased charge carrier separation across the interface of the two materials, and increase selectivity and reaction kinetics of the co-catalyst towards NOx conversion.
An alternative aproach is to enhance the activity of TiO2 through forming a composite with a more narrow bandgap semiconductor. In this project, we will also explore the use of the more narrow bandgap, and 2D material, molybdenum disulfide. A range of synthesis methods to produce the molybdenum disulfide flakes and composite materials will be systematically explored.

NOx Remediation - Developing Composite Photocatalysts That Use Ambient Sunlight to Selectively Reduce NOx Pollution Into N2
The main sources of air pollution in large city areas are exhaust fumes from vehicles and the burning of fuel in industrial processes, which have major consequences for the environment. An increase in the atmospheric concentration of NO2, a key primary air pollutant, has led to an increase in global health problems as they can react to form highly toxic and carcinogenic compounds. High concentrations of NO2 can result in severe plant damage and decreased crop yield. Daily NO2 gas levels in several large cities, including London, often exceed the safety limits set by their governments. Therefore, it is crucial to lower the level of highly harmful NOx in the atmosphere. The primary method for decreasing NOx emissions focus on emission control, while secondary approaches either reduce NOx to N2 or oxidise it to HNO3.

TiO2, one of the most well-known photocatalysts, has been extensively investigated for its ability to remediate atmospheric NOx. Unfortunately, the activity of TiO2 is limited by its wide optical bandgap, that can only utilise a small portion of the solar spectrum (~4% of the photon flux). Recent research has demonstrated that combining TiO2 with other metal oxides to create a composite can significantly increase the selectivity of the reduction of NOx. Therefore, one part of this study will involve the fabrication of novel light-activated coatings that can harvest more sunlight, and thereby increase the efficiency of the photoreaction. Another part of this study will focus on developing catalysts that can drive the selective reduction of NOx into N2. These objectives will be achieved by exploring a range of novel composite catalyst compositions, synthesised by a range of scalable methods.
NOx Remediation - Using Plasmonic Metals to Improve the Activity of Self-Cleaning Windows for Remediating Air Pollution
Cities are becoming increasingly challenging places to live. Emissions from car exhausts release carbon particles and NOx gases that cause respiratory disease and cancer. In some cities, this problem is so severe that breathing city air is likened to smoking 10 cigarettes a day. In many areas of London, NO2 gas levels breach the safety limit of 200 µg.m-3 on a daily basis. NOx gases also cause problems in rural areas, as they react to form highly toxic and carcinogenic compounds that kill plants and reduce crop yields.

Commercial building materials, which possess coatings that use sunlight to convert NOx gas into benign nitrates and improve air quality have recently entered the market. Currently, all of these products rely on the light-activated material, TiO2. Although TiO2 is a highly robust, cheap and active photocatalyst, its wide bandgap limits activity; where only ultraviolet light has sufficient energy to excite the material, which accounts for <5 % of the solar spectrum in power terms.
The aim of this project is to improve the activity of TiO2 coatings produced using the industry method; chemical vapour deposition. The coatings can be improved by the growth of plasmonic metals within or at the surface of the TiO2 coating. Plasmonic metals can enhance the activity of photocatalysts such as TiO2 in several ways. In this project we will explore the incorporation of various plasmonic metals such as Au, Ag and Cu, to achieve this. We will investigate the effects of nanoparticle size, shape and surface plasmon resonance frequency on the activity of their window coatings for remediating polluted air, using our Air Pollution Simulation Chamber.
Water Splitting - Developing Combinatorial Synthesis and Screening Methods for Materials Discovery and Optimisation for Solar Water Splitting
Excessive CO2 emissions from the combustion of fossil fuels is one of the biggest contributors to the greenhouse effect, which is causing a multitude of global problems including unpredictable weather patterns. Carbon neutrality targets have been proposed by various countries, where the UK government made it legally binding to reduce their CO2 emissions to net zero by 2050.
However, the restricted use of fossil fuels exacerbates the energy crisis and necessitates the finding of economic, efficient, and clean alternatives. The advancement of appropriate use the solar energy attracts great attention to solve the energy crisis, since solar power is almost inexhaustible. In addition, solar energy can be converted and stored in chemical bonds by efficient photoelectrochemical water splitting, the clean energy (i.e., hydrogen) generated in the process can satisfy day and night energy demands.
To date, most progress in photoelectrochemical water splitting has been through the systematic study of materials using a “trial and error” method. The most widely studied class of material are the metal oxides, because of their durable, non-toxic and earth abundant properties; with many promising compositions being identified. The performance of these materials can be further improved by forming heterojunctions or elemental doping.

Inspired by the experience of combinatorial chemistry in functional materials discovery, researchers in past few decades have developed promising methods based on combinatorial chemistry and high-throughput screening measurement for the scalable synthesis of a series of materials and study their physical and photoelectrochemical properties. On the other hand, although the application of chemical vapour deposition (CVD) is one of the most popular methods to synthesize photoelectrodes with metal oxide coatings, studies which use combinatorial CVD technology to discover and optimize novel materials with high photoelectrochemical water splitting performance are rarely found.

In this project, a novel combinatorial CVD method will be designed and applied to build several sample libraries for photoelectrochemical water splitting activity comparison. Each library including various discrete metal-oxide based samples (~hundreds) with individually unique composition, structure, and layer thickness will be built in a single synthesis (~hours). The charge carrier behaviour and photoelectrochemical water splitting activity of these samples will be measured by developed high throughput photoelectrochemical microscopy. Computer aided methods (machine-learning tools) will be used to analyse the data produced during the project, in order to study the links between physical properties and water splitting activity, and to develop design rules for the development of high performance materials. Furthermore, the practical tests of discovered and optimised materials on photoelectrochemical water splitting are likely to be conducted in a conventional water splitting test device, to verify the rationality of assessed materials. The main objectives of this project are to find out feasible materials with high-performance in photoelectrochemical water splitting activity by using efficient combinatorial method, and to build up precise mathematical relationship between material physical properties and water splitting performance.
Water Splitting - Developing Solar Water Splitting Prototypes for the Low-Cost Generation of Renewable H2 Fuel
The release of CO2 from the combustion of fossil fuels is the primary cause of Global Warming, which is causing pervasive and lasting damage to the Earth’s climate and ecosystems. To mitigate the potentially catastrophic effects of climate change, an immediate and extensive reduction in CO2 emissions must occur; with the UK pledging to reach net-zero CO2 emissions by 2050. Natural photosynthesis is the perfect example how sunlight can be used to produce renewable carbon-neutral fuels. Bio-inspired approaches (artificial photosynthesis) have shown great promise. One particularly promising approach is solar-driven water splitting to produce clean hydrogen fuel.
Many metal oxide semiconductors are capable of water splitting. They are often quite durable, possess low toxicity and can be grown by low-cost methods. Recent research has shown that a very promising method, perhaps vital for producing the most efficient devices, is to couple metal oxides and form a heterojunction.
During this project we will explore one of the most promising heterojunction systems - WO3/ BiVO4 - using chemical vapour deposition (CVD; a process used by industry to grow coatings at scale). In this project, we will scale the CVD synthesis of WO3/ BiVO4 photoanodes and explore routes to their further enhancement in performance. This will include the study of testing conditions, such as electrolyte composition and concentration, as well as the addition of surface co-catalysts, such as Ni:Fe-OOH, CoPi and CoOx, that have been shown to improve the performance and lower the voltage requirements of the system.

We will characterise the physical properties of their materials using a variety of methods, including: X-ray diffraction, Raman spectroscopy, UV-visible absorption spectroscopy, scanning electron microscopy etc. We will measure the photoelectrochemical water splitting performance of these photoelectrode materials in a purpose built prototype using simulated solar light and outdoors. Through collaboration technoeconomic and life-cycle analysis will be carried out using data generated from the project to estimate the price of H2 that can be delivered using this technology, and provide insight on the components of the system that require further optimisation.




